Departures from the Guide
The public identified departures from the Guide for the Care and Use of Laboratory Animals (Guide) as an area to reduce administrative burden.
In the report Reducing Administrative Burden for Researchers: Animal Care and Use in Research, developed in response to the 21st Century Cures Act, OLAW committed to clarifying the guidance for the reporting requirements of departures from the Guide.
Request for Information
The NIH Office of Laboratory Animal Welfare (OLAW) proposed updates to NOT-OD-12-148 for Departures from the Guide through a Request for Information (RFI) between July 20 and November 1, 2021.
The proposed changes can be found here: NOT-OD-21-161
The comment period is now closed.
The final guidance will be published along with an updated webpage once comments have been considered. Until comments have been considered and new guidance has been finalized, OLAW expects institutions to comply with the guidance in NOT-OD-12-148.
Policies and Laws

PHS Policy IV.A.1. “The Assurance shall fully describe the institution's program for the care and use of animals in PHS-conducted or supported activities. The PHS requires institutions to use the Guide for the Care and Use of Laboratory Animals (Guide) as a basis for developing and implementing an institutional program for activities involving animals.2"
PHS Policy IV.B.3. “As an agent of the institution, the IACUC shall with respect to PHS-conducted or supported activities: prepare reports of the IACUC evaluations conducted as required by IV.B.1. and 2. of this Policy, and submit the reports to the Institutional Official; 8
- The reports must contain a description of the nature and extent of the institution’s adherence to the Guide and this Policy and must identify specifically any departures from the provisions of the Guide and this Policy, and must state the reasons for each departure.”
PHS Policy IV.F.3. “The IACUC, through the Institutional Official, shall promptly provide OLAW with a full explanation of the circumstances and actions taken with respect to:
- Any serious or continuing noncompliance with this Policy;
- Any serious deviation from the provisions of the Guide,13 or
- Any suspension of an activity by the IACUC."

Guidance
| Notice Number | Description | Date |
|---|---|---|
| NOT-OD-21-161 | Request for Information (RFI) on Clarifying the Reporting Requirements for Departure from the Guide for the Care and Use of Laboratory Animals | July 20, 2021 |
| NOT-OD-12-148 | Guidance on Departures from the Provisions of the Guide for the Care and Use of Laboratory Animals | September 10, 2012 |
Quick References
Definitions
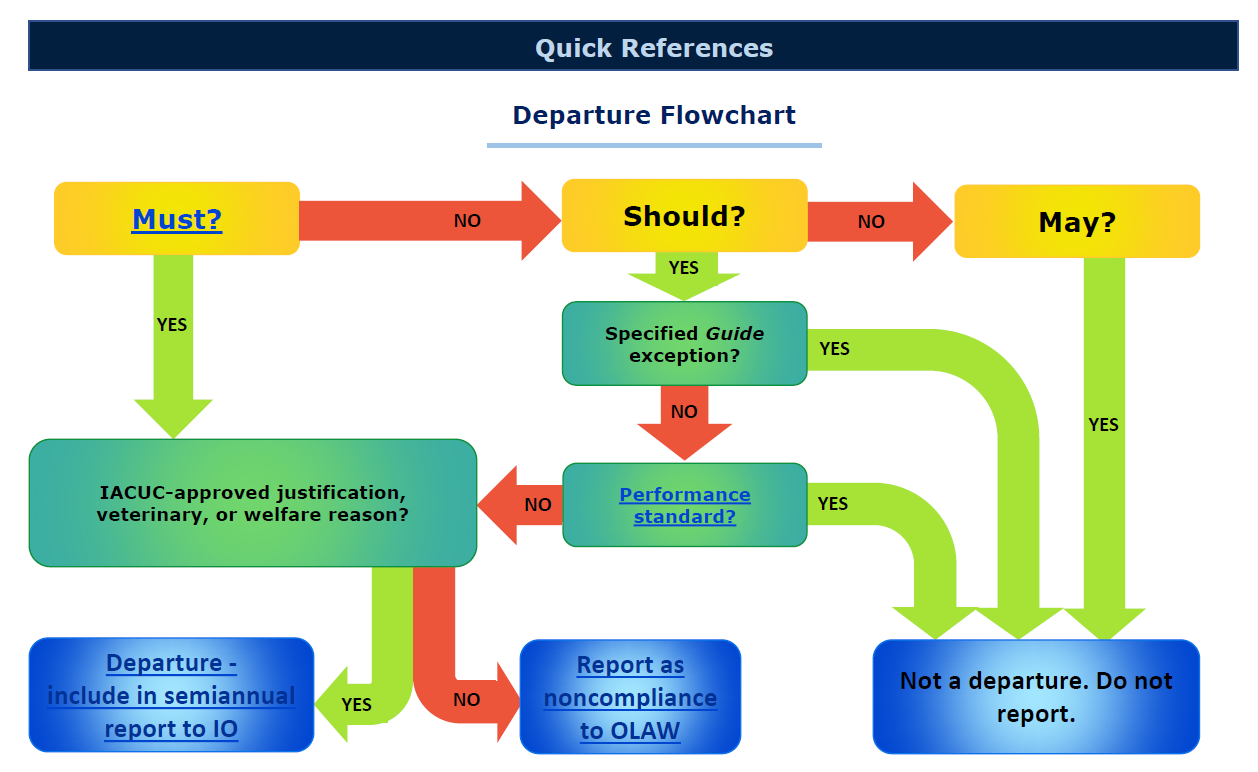
Must, Should, May
The provisions of the Guide are stated in terms of 3 categories of standards for humane animal care and use.
Must: a minimum standard required of all Assured institutions
Should: strong recommendations that can be achieved with an alternative method
May: suggestions that institutions can choose to implement if suitable for their program
Reporting Requirements Table
| Type of Deviation | Status | Report to IO | Report to OLAW |
|---|---|---|---|
Should statement:
| Not a departure | No | No |
Must statement with IACUC-approved:
| Approved departure | Yes, in semiannual report | No |
Should statement:
| Approved departure | Yes, in semiannual report | No |
Deviation from a should statement without:
| Noncompliance [unapproved departure] | Yes, promptly | Yes, promptly |
Deviation from a must statement without:
| Noncompliance [unapproved departure] | Yes, promptly | Yes, promptly |
Departures Flowchart
This is an image. To access flowchart hyperlinks, click on the flowchart, which will open in a new tab. You may then access the hyperlinks.
Examples Based on Reporting Category
Reporting to the Institutional Official (IO) is not required:
May statements in the Guide are suggestions that institutions can choose to implement if suitable for their program. These deviations are not included in the semiannual report to the IO.
When deviating from a specifically described Guide exception for should statements.
The Guide establishes exceptions for specific should statements, which are not departures from the Guide. There are no Guide exceptions for must statements.
Example: A PI requests single housing for her rats to measure individual urine output post-treatment with a test article. After considering the specific details of the proposed experiment, the IACUC approves the protocol.
Guide: "Social animals should be housed in stable pairs or groups of compatible individuals unless they must be housed alone for experimental reasons or because of social incompatibility" (page 51).
Discussion: This is a deviation from a should statement according to a specifically established exception in the Guide (experimental reasons). Exceptions are not departures from the Guide and need not be reported in the semiannual report to the IO.
When deviating from a should statement using established performance standards.
Performance standards can only be applied to should and not must statements.
Example 1: A PI proposes to forgo cage sanitization for a 5-week study because animals are exposed to a biohazardous infectious agent. An SOP was developed based on data collected at the institution addressing cage density, parameters for sanitary conditions, regular ammonia level testing, and use of specialized bedding that facilitates cleanliness. Both the SOP and protocol were reviewed and approved by the IACUC.
Guide: "In general, enclosures and accessories, such as tops, should be sanitized at least once every 2 weeks" (page 70).
Discussion: This is a deviation from a should statement that follows a performance standard using data established at the institution. It is not a departure from the Guide and is not reported in the semiannual report to the IO.
Example 2: All mouse wire bar lids are sanitized once every 4 weeks according to performance standards established by the institution. The SOP was reviewed and approved by the IACUC.
Guide: "In general, enclosures and accessories, such as tops, should be sanitized at least once every 2 weeks" (page 70).
Discussion: This is a program-wide institutional, rather than a study-specific deviation from a should statement that follows a performance standard using data established by the institution. It is not a departure from the Guide and is not reported in the semiannual report to the IO.
Reporting to the Institutional Official (IO) is required:
Example: The PI proposes to confine dogs for 3 days in small cages to limit the dogs' ability to stand as part of a scientifically justified research requirement. The IACUC reviews and approves the scientific justification and the protocol.
Guide: "At a minimum, animals must have enough space to express their natural postures..." (page 56).
Discussion: This is a deviation from a must statement of the Guide that has been scientifically justified by the PI and reviewed and approved by the IACUC. It is an approved departure and must be reported in the semiannual report to the IO.
Example: In describing a food-restricted animal study, the PI stated that animals must be anesthetized before weighing and cited data demonstrating the adverse effect of anesthesia on the animals' performance of behavioral tasks required in the proposed study. The research team will monitor and weigh the animals and requested permission to weigh monthly rather than weekly. The IACUC reviewed and approved the request.
Guide: Regarding food-restricted diets, the Guide states: "Body weights should be recorded at least weekly" (page 31).
Discussion: This is a deviation from a should statement based on scientific justification that has been reviewed and approved by the IACUC. It is an approved departure from the Guide and must be reported in the semiannual report to the IO.
Reporting to OLAW is required:
Example: The IACUC learns that one of the satellite facilities has inadvertently been omitted from the disaster plan.
Guide: "Facilities must therefore have a disaster plan" (page 35).
Discussion: This is a noncompliance. The IACUC must report to OLAW through the IO and develop a plan and schedule to correct this unapproved deviation from a must statement in the Guide.
Example 1: The IACUC approved a PI's protocol allowing the research team to sanitize solid-bottom rodent cages weekly in a satellite facility. The PI goes on sabbatical and the research team decides that sanitation every 3 weeks is sufficient. A post-approval monitor discovers the discrepancy and informs the IACUC.
Guide: "In general, enclosures and accessories...should...be sanitized at least once every 2 weeks. Solid-bottom caging...usually require[s] sanitation at least once a week" (page 70).
Discussion: This is a noncompliance. The IACUC must report to OLAW through the IO and develop a plan and schedule to correct this unapproved deviation from a should statement in the Guide.
Example 2: An institution has begun a breeding program for Siberian hamsters, a new species at the institution. The visiting investigator who was charged with setting up a breeding program assured the IACUC members that the species does well with trio breeding. The IACUC approved the protocol without considering that the facility's cages have 20 inches2 of floor space, without consulting the investigator, veterinarian, or the literature regarding space requirements.
Guide: "The space recommendations presented here are based on professional judgement and experience. They should be considered the minimum for animals housed under conditions found in laboratory animal housing facilities" (page 56 and Table 3.2).
Discussion: This is a noncompliance. Breeding in space that did not meet Guide standards was permitted without appropriate justification or use of a well-established performance standard regarding space requirements. The IACUC must report to OLAW through the IO and develop a plan and schedule to correct this unapproved deviation from a should statement in the Guide.
Example 3: The PI for a guinea pig study is trying to cut costs and instructed his laboratory manager to order non-pharmaceutical-grade euthanasia drugs for his study. The original animal activity approved by the IACUC specified pharmaceutical grade drugs and the PI did not submit an amendment to the IACUC requesting the change.
Guide: "The use of pharmaceutical-grade chemicals and other substances ensures that toxic or unwanted side effects are not introduced into studies conducted with the experimental animals. They should therefore be used, when available, for all animal-related procedures. The use of non-pharmaceutical-grade chemicals or substances should be described and justified in the animal use protocol and be approved by the IACUC" (page 31).
Discussion: This is a noncompliance because it is a change that was not approved by the IACUC in advance of implementation, and therefore constitutes a deviation from an IACUC-approved protocol. The IACUC must report to OLAW through the IO and develop a plan and schedule to correct this unapproved deviation from a should statement in the Guide.
Resources
FAQs
FAQ C.7 What are PHS Policy reporting requirements for departures from the Guide?
The IACUC must review and approve departures from the Guide. IACUC approval of departures from the Guide must be based on scientific, veterinary medical, or animal welfare issues. Semiannual reports from the IACUC to the Institutional Official (IO) must identify specifically any departures from the Guide (PHS Policy IV.B.3.). Read the Guide carefully; it establishes exceptions in specific situations; these are not departures from the Guide and are not required to be reported to the IO.
Guidance in the Eighth Edition of the Guide is stated in terms of standards that must be met, standards that should be met, and standards that may be met.
FAQ G.10 What is OLAW’s position on performance standards?
OLAW encourages the cooperative application of a range of expertise to develop outcome-based performance standards that enhance the quality of animal care and use programs. OLAW does not consider a performance standard to be a departure from the Guide. A well-developed performance standard meets the following criteria:
- supports scientific objectives;
- supports the health and welfare of the animal;
- includes a justified performance index; and
- has associated outcome criteria.
IACUCs are able to meet their responsibility to ensure humane animal care and use while advancing quality scientific research through the use of performance standards.
Webinars
| Webinar Link | Webinar Date |
|---|---|
| Performance Standards | April 19, 2012 |
| Departures from the Guide | October 4, 2012 |
Articles
| Title | Description | Date |
|---|---|---|
| Design, Implementation, Monitoring, and Sharing of Performance Standards for Laboratory Animal Use | Summary of a workshop by the Institute for Laboratory Animal Research Roundtable on Science and Welfare in Laboratory Animal Use, published by The National Academies Press. | 2015 |