Reporting Noncompliance
One of the actions identified in the report Reducing Administrative Burden for Researchers: Animal Care and Use in Research in response to the 21st Century Cures Act is to provide clarification on noncompliance reporting. In NOT-OD-25-148, OLAW provides additional examples of reportable situations, situations where reporting is not normally required, the time frame for reporting, and the information to be reported.
Note that the information on this site may not specifically address your institution's unique concerns. A preliminary call to OLAW to discuss any questions is always encouraged.
Get Started
OLAW accepts reports and allegations of noncompliance from institutions and individuals. All of these reports are evaluated by OLAW and substantiated allegations may result in a range of actions by the Office. Read more to learn about noncompliance reports, including what to report, when to report, how OLAW evaluates reports, and potential resulting actions. Although possible, severe compliance actions affecting an award are rare because institutions are usually able to address incidents successfully and take appropriate actions to prevent reoccurrence.
Policies and Laws

Health Research Extension Act of 1985 (Public Law 99-158):
"(d) If the Director of NIH determines that...
- the conditions of animal care, treatment, or use in an entity which is receiving a grant, contract, or cooperative agreement involving research on animals under this title do not meet applicable guidelines established under subsection (a);
- the entity has been notified by the Director of NIH of such determination and has been given a reasonable opportunity to take corrective action; and
- no action has been taken by the entity to correct such conditions;
the Director of NIH shall suspend or revoke such grant or contract under such conditions as the Director determines appropriate."
- "The IACUC, through the Institutional Official, shall promptly provide OLAW with a full explanation of the circumstances and actions taken with respect to:
- any serious or continuing noncompliance with this Policy;
- any serious deviation from the provisions of the Guide;
- any suspension of an activity by the IACUC."
- "The IACUC may suspend an activity which it previously approved if it determines that the activity is not being conducted in accordance with applicable provisions of the Animal Welfare Act, the Guide, the institution's Assurance, or IV.C.1.a.-g. of [the PHS] Policy. The IACUC may suspend an activity only after review of the matter at a convened meeting of a quorum of the IACUC and with the suspension vote of a majority of the quorum present."
- "If the IACUC suspends an activity involving animals, the Institutional Official in consultation with the IACUC shall review the reasons for suspension, take appropriate corrective action, and report that action with a full explanation to OLAW."

Guidance
| Notice Number | Description | Date |
|---|---|---|
| NOT-OD-25-148 | Notice on Update to Guidance on Prompt Reporting to OLAW Under the PHS Policy on Humane Care and Use of Laboratory Animals (replaces the rescinded Notice NOT-OD-05-034) | January 20, 2026 |
| NOT-OD-13-044 | Notice of Change to Electronic Submission of Final Noncompliance Reports to the Office of Laboratory Animal Welfare | February 21, 2013 |
| NOT-OD-10-081 | Guidance on Confirming Appropriate Charges to NIH Awards During Periods of Noncompliance for Activities Involving Animals | April 15, 2010 |
| NOT-OD-07-044 | NIH Policy on Allowable Costs for Grant Activities Involving Animals When Terms and Conditions are not Upheld | January 26, 2007 |
Quick References
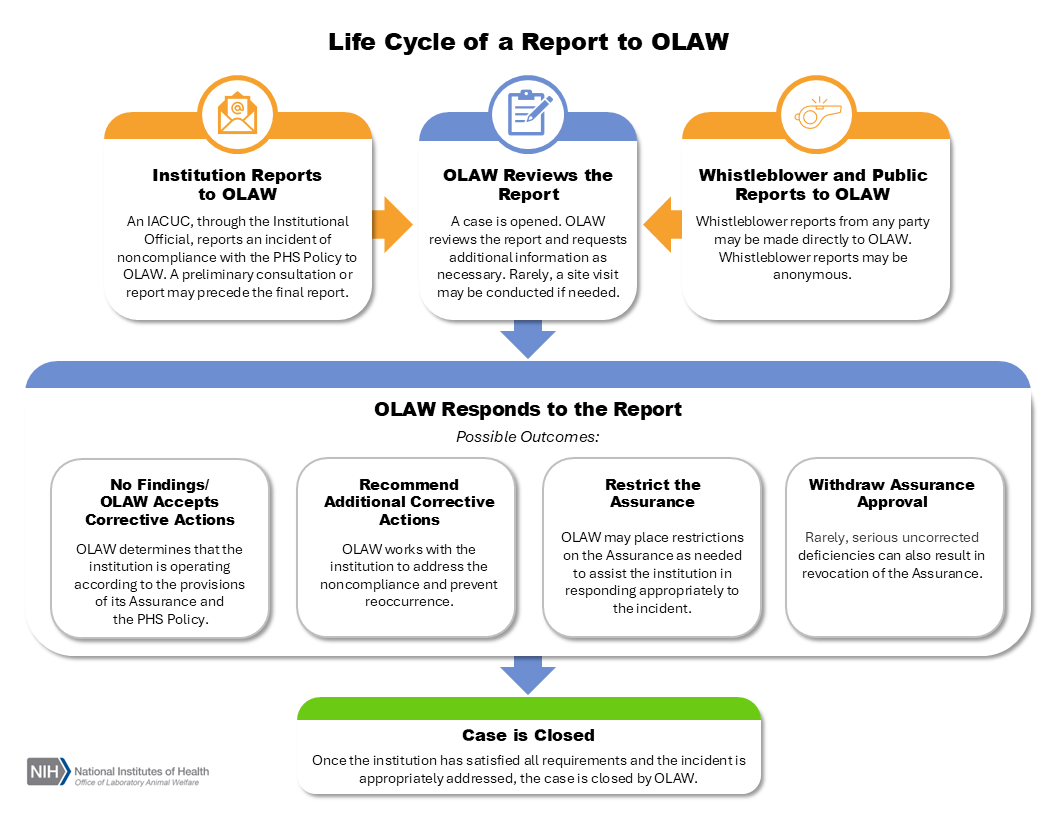
Life Cycle of a Report to OLAW
1. OLAW Receives a Report
Reports arrive at OLAW's Division of Compliance Oversight in one of two ways: either they are submitted by an institution as a self-report, or they are directly communicated to OLAW by a third party. (For guidance on institutional responsibilities regarding whistleblowers and whistleblower protections, see FAQ. G.14.) If submitted by an institution, information should be communicated as a preliminary report via phone or email as soon as possible, even if an investigation is ongoing. This allows OLAW to provide assistance throughout the investigation and corrective process, especially if it is expected to be protracted. The final report can be submitted once additional information has been gathered and corrective actions have been determined.
2. OLAW Reviews and Responds to the Report
OLAW reviews all reports and allegations of noncompliance. Generally, questions can be resolved through dialogue between OLAW and the institution. If a whistleblower or public report is received, institutions will be notified of the nature of the complaint and, except in rare circumstances, have the opportunity to undertake corrective actions. If OLAW is unable to substantiate the allegations in the report after review and a request for additional information, or if the case is substantiated and the institution has implemented appropriate corrective actions, the case is closed by OLAW. If additional straightforward corrective actions are necessary before the case can be closed, OLAW works with the institution to identify and implement any remaining steps to address the noncompliance and prevent recurrence.
Though uncommon, OLAW may take additional action to assist the institution in responding appropriately to the incident, such as:
- Placing the institution on an enhanced reporting schedule, where regular follow-up reports are required to demonstrate continued effectiveness of corrective actions;
- Performing further in-depth review via a site visit;
- Restricting the Assurance applicability for some activities;
- Requiring OLAW review of all Assured activities, etc.
Rarely, serious uncorrected deficiencies can also result in revocation of the Assurance. However, compliance actions affecting an award are rare because institutions are usually able to address incidents successfully and take appropriate actions to prevent reoccurrence.
3. The Case is Closed
Once the institution has satisfied all requirements and the incident is appropriately addressed, the case is closed by OLAW.
For additional information regarding OLAW's process of evaluation, including a detailed explanation of the sequence of events and outcomes of OLAW's review, see the memo "Compliance Oversight Procedures."

Compliance Review Process Flowchart
A flowchart of the compliance review process can be accessed by clicking the thumbnail. The expanded image will open in your browser.
A downloadable PDF version of the flowchart is available by clicking the button below.
Examples Based on Reporting Category
Institutions are reminded that the requirements for reporting extend to all live, vertebrate animal activities covered by the PHS Policy. Those institutions whose Assurance states that all incidents will be reported as required regardless of funding source must report accordingly. While the IACUC has the duty to report based on consideration of the circumstances, OLAW reserves the right to make the final determination regarding the reportability of an event. For this reason, a preliminary call to OLAW is always encouraged if there are questions about reporting.
Situations that meet the provisions of section IV.F.3 and are identified by external entities such as the United States Department of Agriculture (USDA) or AAALAC International, or by individuals outside the IACUC or outside the institution, are not exempt from reporting.
Use the dropdowns below to explore when reporting is required.

As a comprehensive list of definitive examples of reportable situations is impractical, the examples below do not cover all instances but demonstrate the threshold at which OLAW expects to receive a report. Items not listed may still qualify for reporting. Institutions should use rational judgment in determining what situations meet the provisions of PHS Policy section IV.F.3. and fall within the scope of the examples below and consult with OLAW if in doubt. OLAW welcomes inquiries and discussion and will provide guidance regarding specific situations.
The following text is taken from NOT-OD-25-148:
| IACUC Function and Protocol Review | Animal Management | Program Management |
|---|---|---|
|
|
|
| IACUC Function and Protocol Review | Animal Management | Program Management |
|---|---|---|
|
|
|
Submitting Noncompliance Reports
Preliminary reports may be made via phone or email to the Division of Compliance Oversight. All final institutional self-reports should be sent via email, through the IO, in PDF format (see NOT-OD-13-044). The signature of the IO provides verification that this requirement is met. Use the dropdowns below to identify what and when to report.
Preliminary self-reports provide a flexibility for institutions to meet the requirement for prompt reporting even though it may take time to fully investigate reportable circumstances and implement appropriate corrective actions. In addition, this flexibility allows OLAW to work with institutions during the investigative process to determine if the circumstances meet the requirements for reporting and provide guidance prior to receipt of a formal written report. Include as many of the following items as possible in the preliminary report to OLAW (the following list taken from NOT-OD-25-148):
- Assurance number
- Award numbers for National Science Foundation (NSF) activities. Only NSF award numbers must be included in the report. Other award numbers need not be included unless requested by OLAW or if determined to be applicable by the reporting institution.
- Funding source for all PHS supported activities, including those having an MOU with OLAW. E.g., NIH, NSF, National Aeronautics and Space Administration (NASA), U.S. Department of Veterans Affairs (VA)
- Complete explanation of the situation, including what happened, when and where, species of animal(s) involved, and category of individual(s) involved (e.g., Principal Investigator or Co-Principal Investigator, technician, animal caretaker, student, veterinarian, etc.)
- A full description of any situation, that in the judgment of the IACUC and Institutional Official, may be a threat to PHS-supported activities
- Description of actions taken by the institution to address the situation
- Description of short- or long-term corrective plans and implementation schedule(s)
The final report should identify the date of the preliminary report, address any of the above not included in the preliminary report, and provide any updates or changes since the preliminary report, if made.
Click here to view an example final report
Institutions should notify OLAW promptly, i.e., without delay. Because the time required to fully investigate and devise corrective actions may be lengthy, OLAW recommends that an authorized institutional representative provide a preliminary report to OLAW as soon as possible and follow-up with a thorough report once action has been taken. Reports should be submitted as situations occur and not collected and submitted in groups or with the Annual Report to OLAW.
In addition to self-reports from institutions, OLAW accepts concerns from other interested parties, including whistleblower and anonymous reports. Whistleblower and anonymous reports should be submitted by phone or email to the Division of Compliance Oversight. Although OLAW will accept a whistleblower's report, OLAW encourages all parties to first consider using institutional reporting processes for animal welfare concerns. OLAW may withhold identifying information to protect whistleblowers; however, individuals should be aware that they may still be identified by others due to the particular circumstances surrounding the report. Protection from reprisal for whistleblowers must be addressed at the institutional level and according to applicable law. OLAW cannot guarantee that individuals making a report will be protected from adverse institutional actions. See also FAQ. G.14
If the incident was not compliant with the terms and conditions of the award supporting the activities, it may need to be reported to the funding component. Charges may not be made to PHS awards for noncompliant activities, though some costs may be allowable on case-by-case basis in discussion with the funding component (see also NOT-OD-10-081).
FOIA
Institutions and individuals are reminded that case documents submitted to OLAW, including email communications and preliminary reports, may be publicly available under the Freedom of Information Act (FOIA) once cases are closed. To learn more about the NIH FOIA process, see FAQ C.4..
Resources
FAQs
FAQ C.2 What kinds of situations should be reported to OLAW under IV.F.3. of the PHS Policy, and when, where, and how should they be reported?
FAQ C.3 Should the IACUC report sanctions other than suspensions that are imposed by the Committee or by other institutional officials?
Sanctions imposed by the IACUC or by an institutional official due to serious or continuing noncompliance or serious deviations from the Guide must be reported to OLAW. Guidance on reporting noncompliance is in the NIH Guide for Grants and Contracts NOT-OD-05-034.
FAQ C.4 Are all documents submitted to OLAW subject to the Freedom of Information Act?
The Freedom of Information Act (FOIA), 5 U.S.C.522, provides individuals with a right of access to records in the possession of the federal government. All documents submitted to OLAW are subject to the FOIA. However, the government may withhold information pursuant to the exemptions and exclusions contained in FOIA. To learn more about the NIH FOIA process, see the OLAW Online Seminar: Openness and Transparency and Biomedical Research Oversight.
In a ruling by the US District Court for the District of Columbia Div. No 99-3024, In Defense of Animals v. Department of Health and Human Services (HHS), 9/28/2001, the Court ruled that HHS (NIH’s parent organization) may withhold IACUC members’ names. NIH does release the names of the IACUC Chairperson, veterinarian, and Institutional Official as reported in Assurances.
Note also that footnote 6 in the PHS Policy allows institutions to represent the name of IACUC members other than the chair and veterinarian by using numbers or other symbols, provided there is sufficient information to allow OLAW to determine that appointees are appropriately qualified. Identities of members must be readily ascertainable by the institution and available to OLAW upon request.
In providing the facility and species inventory as part of the Assurance submitted to OLAW, institutions may identify animal areas in any manner, e.g., initials, ID number. It is not necessary to provide OLAW with detailed diagrams of facilities or room numbers, unless specifically requested by OLAW.
Institutions are also advised to consult the Guidance on Prompt Reporting to OLAW under the PHS Policy (NIH Guide for Grants and Contracts NOT-OD-05-034) with respect to what information is expected to be reported when reporting noncompliance. Disciplinary documents (e.g., letters of reprimand) and correspondence between the IACUC and investigators are generally not required by OLAW, although they may be requested.
Additional information about the FOIA, including guidelines for submitting FOIA requests, is available at: https://www.nih.gov/institutes-nih/nih-office-director/office-communications-public-liaison/freedom-information-act-office
FAQ C.5 Are institutions required by FOIA to release information about their research, animal care programs, and IACUCs?
The Freedom of Information Act (FOIA) is a federal law that provides individuals with a right of access to records in the possession or control of the federal government. That means that OLAW is required to release information in its possession or control unless that information is specifically exempted from release as described in section 522(b) of the FOIA. There is no federal requirement for institutions (other than the federal government) to comply with the federal FOIA. State or local laws, sometimes referred to as “Sunshine Laws” may govern the release of information by institutions. These vary widely from state to state. Even within a state, different regulations may govern disclosure for public and private institutions. Institutions are encouraged to seek the advice of their legal counsel regarding disclosure of information.
FAQ C.7 What are PHS Policy reporting requirements for departures from the Guide?
The IACUC must review and approve departures from the Guide. IACUC approval of departures from the Guide must be based on scientific, veterinary medical, or animal welfare issues. Semiannual reports from the IACUC to the Institutional Official (IO) must identify specifically any departures from the Guide (PHS Policy IV.B.3.). Read the Guide carefully; it establishes exceptions in specific situations; these are not departures from the Guide and are not required to be reported to the IO.
Guidance in the Eighth Edition of the Guide is stated in terms of standards that must be met, standards that should be met, and standards that may be met.
FAQ G.14 What are the institution’s responsibilities regarding whistleblowers?
PHS Policy IV.B.4. requires the IACUC to review concerns involving the care and use of animals at the institution. The Guide for the Care and Use of Laboratory Animals (Guide, page 24) states that a program for reporting animal welfare concerns should “include a mechanism for anonymity, compliance with applicable whistleblower policies, nondiscrimination against the concerned/reporting party, and protection from reprisals.” OLAW expects institutions to enforce protections afforded by institutional policy and applicable law for any individual(s), who in good faith, reports an animal welfare concern.
- Once an incident has been reported to the IACUC and verified as a noncompliance, OLAW recommends that an authorized individual at the institution provide a preliminary report as soon as possible (see NOT-OD-05-034). This includes any reports made by whistleblowers and/or allegations of retaliation.
- It is important for institutions to have a feedback mechanism notifying the complainant of the outcome to assure whistleblowers that their concerns have been acknowledged (Guide, page 23-24).
- Whistleblowers are encouraged to use the institution’s internal reporting structure to report animal welfare concerns but may also contact OLAW to either make a report or receive anonymous consultation. OLAW may withhold identifying information to protect whistleblowers but cannot guarantee that individuals making a report will remain anonymous or be protected from adverse institutional actions. Even with identifying information withheld, individuals may be identified by others due to the circumstances surrounding the report.
- Institutions are expected to comply with applicable USDA regulations. The Animal Welfare Regulations require the IACUC, as an agent of the research facility, to review and, if warranted, investigate concerns involving the care and use of animals at the research facility resulting from public complaints or reports from research facility personnel (9 CFR § 2.31(c)(4)). Furthermore, personnel training must include guidance in “methods whereby deficiencies in animal care and treatment are reported ... No facility employee, Committee member, or laboratory personnel shall be discriminated against or be subject to any reprisal for reporting violations of any regulation or standards under the [Animal Welfare Act]” (9 CFR § 2.32 (c)(4)).
Because discrimination and reprisal can discourage individuals from reporting and delay correction of animal welfare concerns, OLAW takes allegations of retaliation seriously. OLAW’s response to allegations of reprisal may include, but is not limited to, confirming that the institution’s policy and process for responding to reports complies with the institution’s Animal Welfare Assurance and the Guide, or additional action as necessary, based on the severity and nature of the allegations.
Webinars
| Webinar Link | Webinar Date |
|---|---|
| Animals in Research: Tips to Keep Out of the Doghouse: 2021 NIH Virtual Seminar Session | November 4, 2021 |
| Adverse Events at Research Facilities | December 7, 2017 |
| Self-Evaluation and Reporting: Always Let the Guide be Your Conscience | December 15, 2016 |
| Reporting Noncompliant Events to OLAW | March 5, 2009 |
| When Terms and Conditions are not Met | December 4, 2008 |
Articles
| Reporting Noncompliance Topic Index | Topic Index section on reporting noncompliance can be found under the heading "Institutional Reporting to OLAW." |
| Terms and Conditions Topic Index | Topic index section with information on the terms and conditions of an award can be found under the heading "Animal Welfare Assurances." |
Documents
| Document Link | Description |
|---|---|
| Compliance Oversight Procedures | This memorandum summarizes OLAW's procedure when conducting compliance oversight activities. |
| Example Final Report | This document provides a fictional example of a noncompliance report. Institutions need not follow the exact format so long as all the required information is clearly presented. |
Contact the Division of Compliance Oversight
Phone: 301-496-7163
E-mail: [email protected]