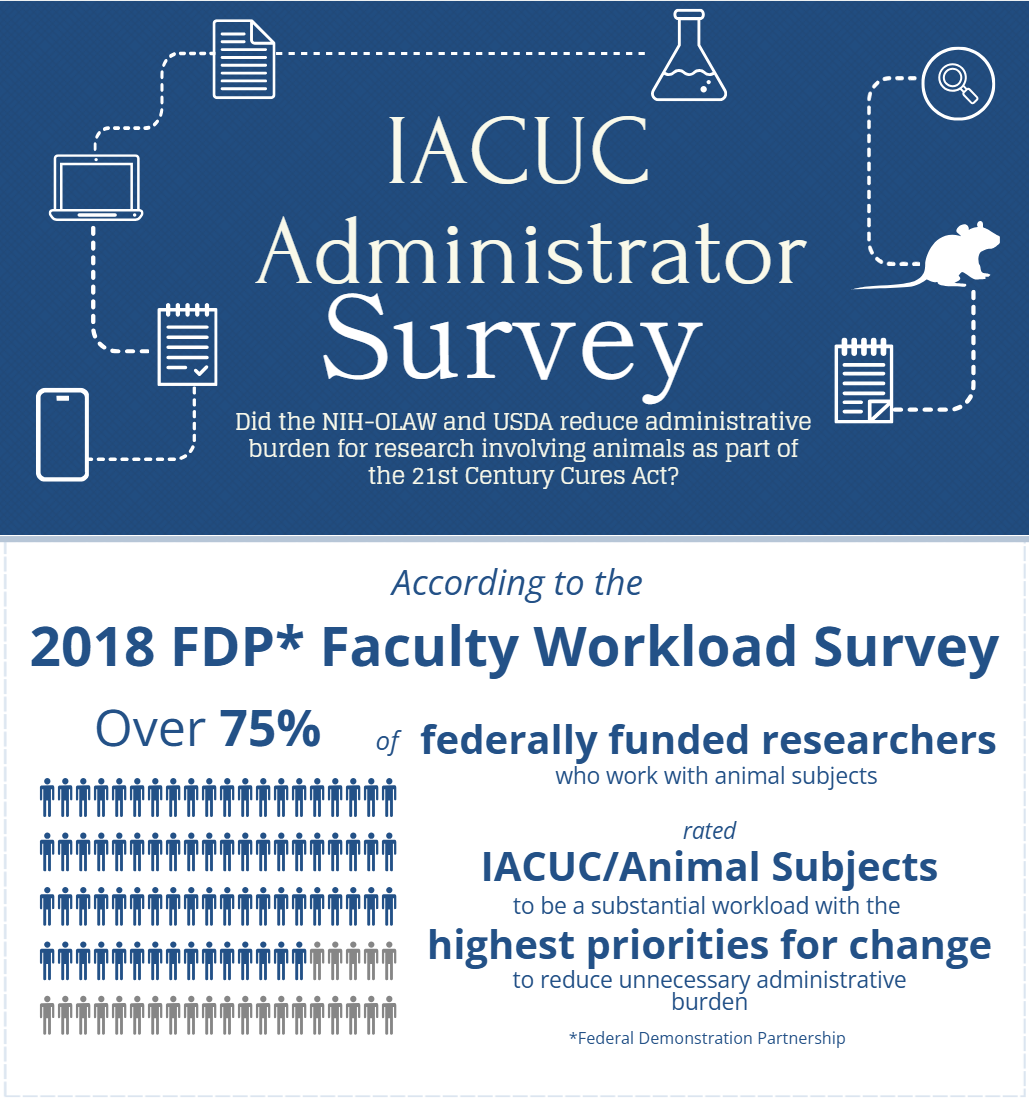
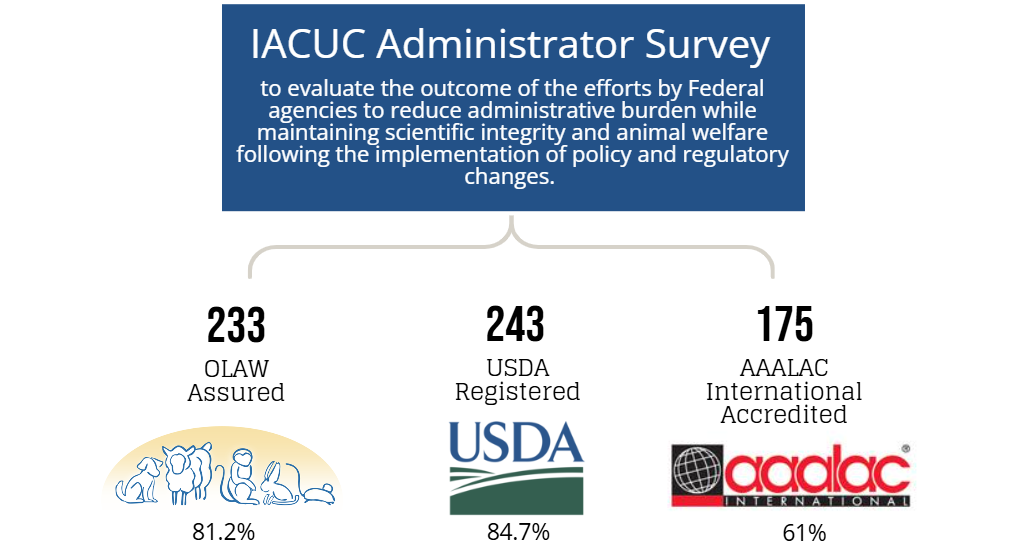
In 2019, the National Institutes of Health (NIH), the United States Department of Agriculture (USDA), and the Food and Drug Administration (FDA) published the report Reducing Administrative Burden for Researchers: Animal Care and Use in Research describing the recommendations of the 21st Century Cures Act, Section 2034(d), Working Group and decisions of the agencies to reduce administrative burden on investigators while maintaining the integrity and credibility of research findings and protection of research animals. One of the recommendations described in this report included the agencies plan to evaluate the outcome of the efforts to reduce administrative burden while maintaining scientific integrity and animal welfare following the implementation of policy and regulatory changes.
A multi-faceted evaluation plan was developed to help the agencies determine whether the implemented policy and regulatory changes were effective at reducing administrative workload, allowing investigators more time to actively focus on their research.
On this page:
Get Started
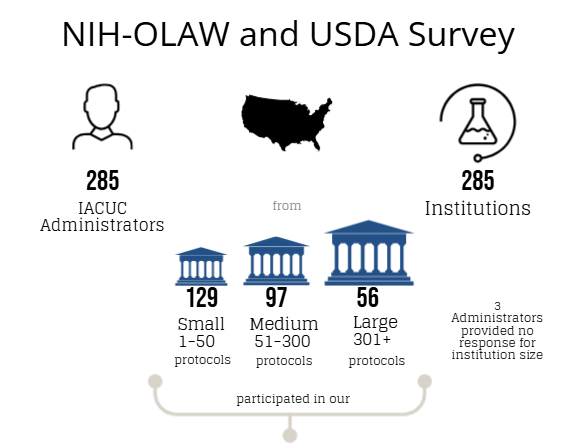
An online Qualtrics survey was distributed from June 1-September 15, 2023, to individuals (e.g., IACUC administrators, coordinators, etc.) whose responsibilities include a direct role in the oversight of their IACUC program and/or IACUC policies and operations at approximately 1,470 OLAW Domestic Assured and USDA-Registered institutions or facilities. The objective of this survey was to determine if:
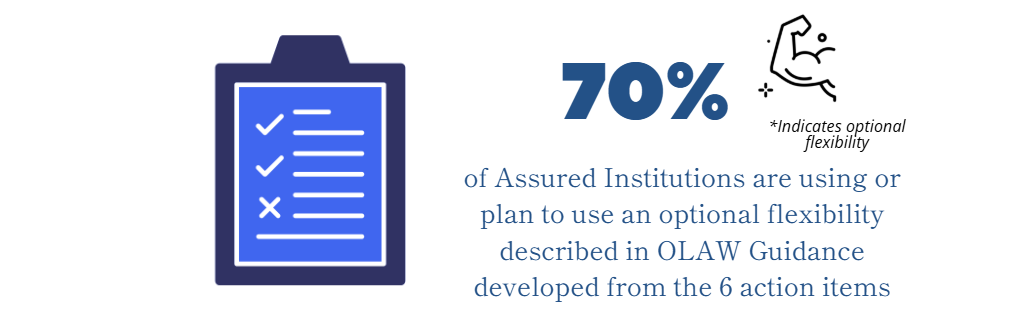
- institutions implemented optional OLAW flexibilities in their animal care and use programs and
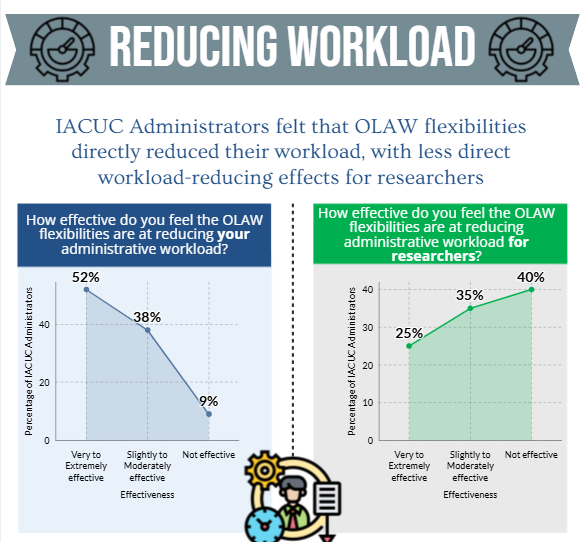
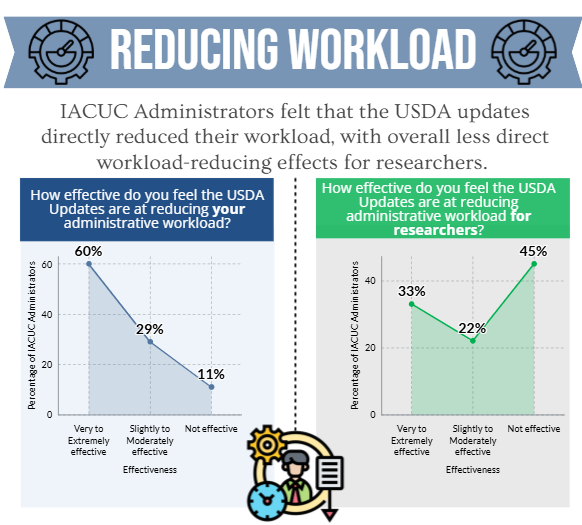
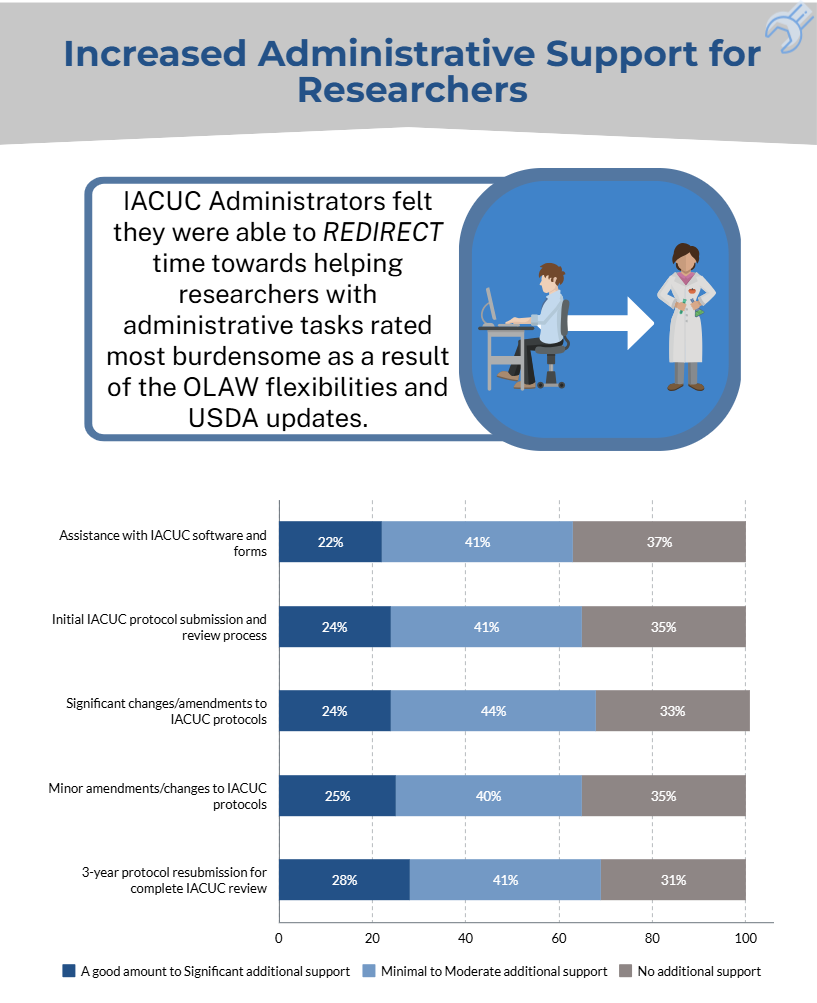
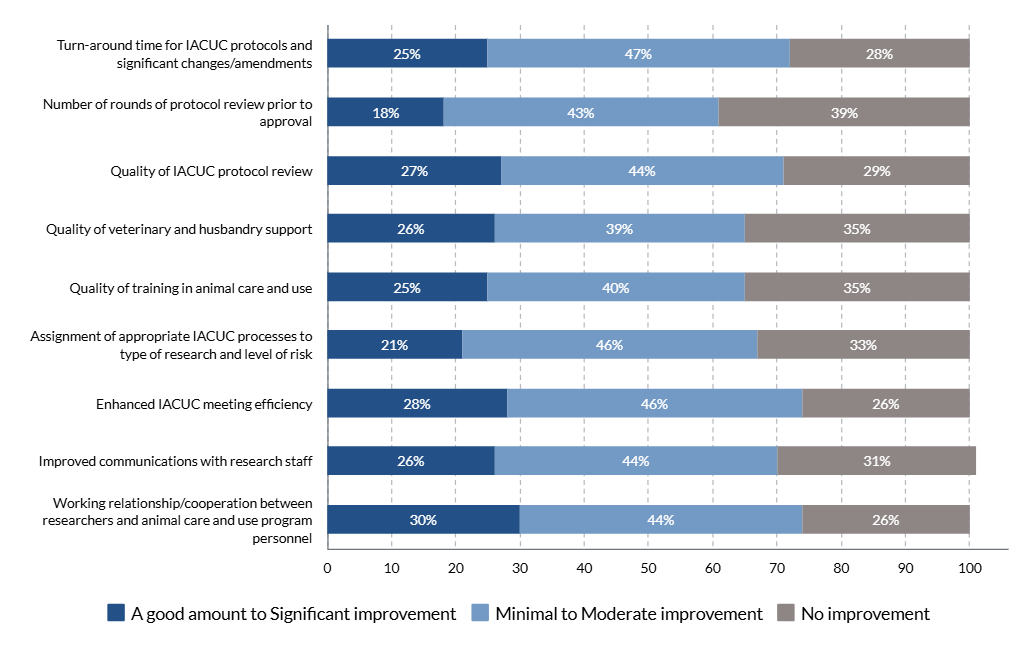
- the OLAW flexibilities and USDA updates directly and/or indirectly reduced administrative workload for administrative staff and investigators.
The results of the IACUC Administrator surveys will help to inform the development of a survey geared towards investigators.
Policies and Laws

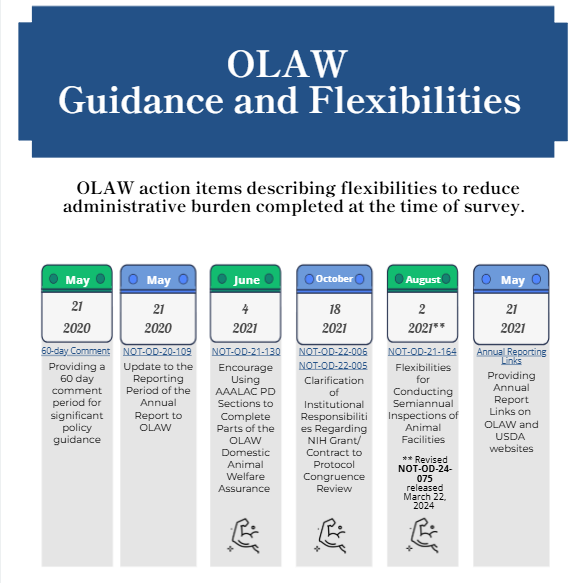
The federal agencies identified several actions to reduce burden and improve coordination of regulations and policies with respect to research activities involving animals. At the time of of the survey (June 2023) OLAW published 5 Guide Notices related to the 21st Century Cures Act that were either mandatory, optional, or required no action from Assured institutions.
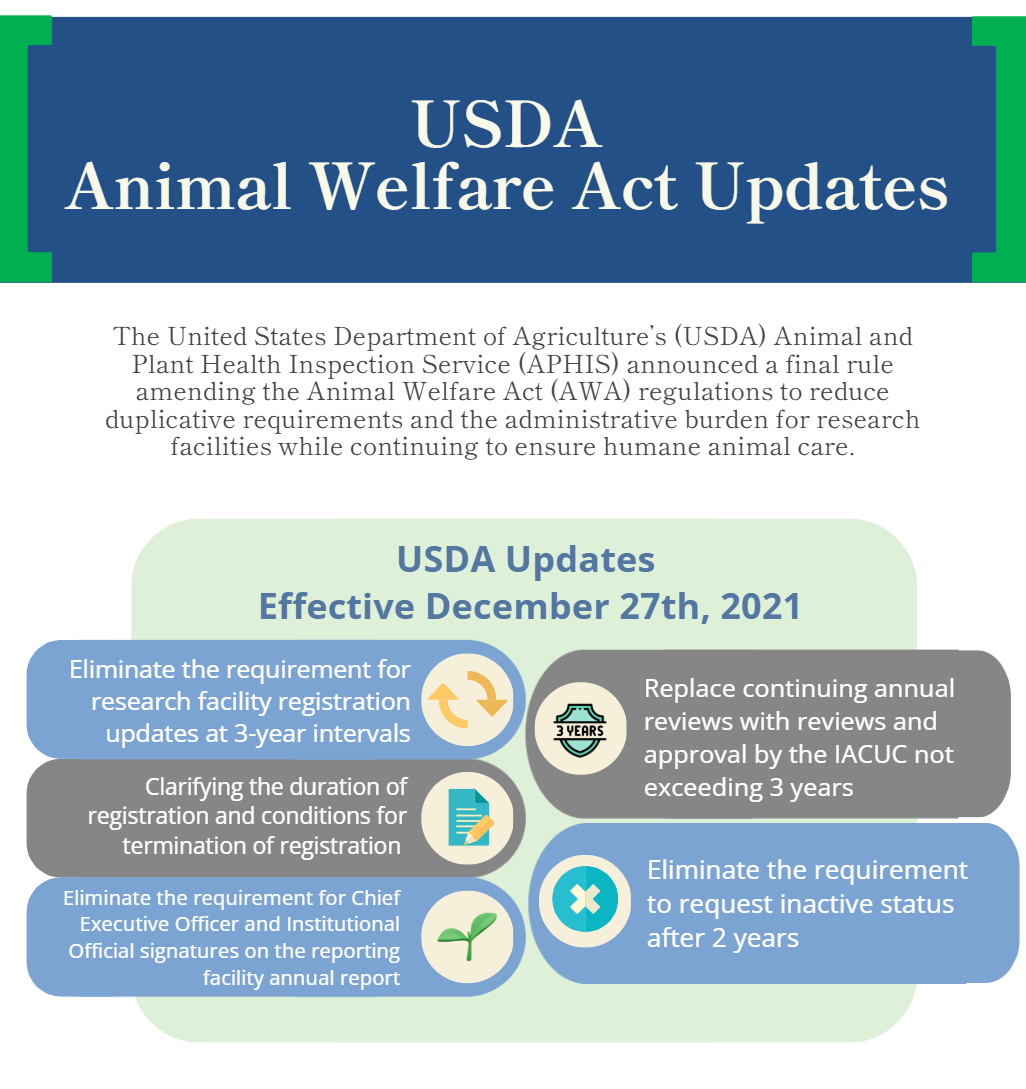
Additionally, as an effort to harmonize requirements across agencies, the USDA amended the Animal Welfare Act (AWA) regulationsto reduce duplicative requirements and the administrative burden for research facilities while continuing to ensure humane animal care, which became effective December 27th, 2021.
Federal Register Notice 2019-18611 - Laboratory Animal Welfare: Report on Reducing Administrative Burden for Researchers: Animal Care and Use in Research
Federal Register Notice 2018-26557 - Laboratory Animal Welfare: Draft Report on Recommendations to Reduce Administrative Burden on Researchers
Federal Register Notice 2021-25614 - AWA Research Facility Registration Updates, Reviews, and Reports

Guidance
| Notice Number | Description | Date | Mandatory, Optional, No Action Required |
|---|---|---|---|
| NOT-OD-20-109 | Notice of Update to the Reporting Period of the Annual Report to OLAW Harmonizing | May 21, 2020 | Mandatory |
| NOT-OD-21-130 | Notice to Encourage Using AAALAC International Program Description Sections to Complete Parts of the OLAW Domestic Animal Welfare Assurance | June 4, 2021 | Optional |
| NOT-OD-22-005 | Notice of Clarification of Institutional Responsibilities Regarding NIH Grant to Protocol Congruence Review | October 18, 2021 | Optional |
| NOT-OD-22-006 | Notice of Clarification of Offeror Responsibilities Regarding Contract to Protocol Congruence Review | October 18, 2021 | Optional |
| NOT-OD-21-164 | Guidance on Flexibilities for Conducting Semiannual Inspections of Animal Facilities Updated March 22, 2024 with NOT-OD-24-075 | August 2, 2021 | Optional |
| Providing Annual Report Links on OLAW and USDA websites | Publicly available July 5, 2022 | No Action Required | |
| 60-day Comment Period |
No Action Required |
Quick References
IACUC Administrator Survey:
(Click the heading to view the dropdown and learn more.)Resources
Webinars
| Webinar Link | Webinar Date |
|---|---|
| 21st Century Cures Act: Updates and Your Questions Answered | March 10, 2022 |
| 21st Century Cures Act: Update on Implementation | March 11, 2021 |
| 21st Century Cures Act: Next Steps | December 5, 2019 |
Questions?
Call (301-496-7163) or e-mail ([email protected]) OLAW for answers to your questions.